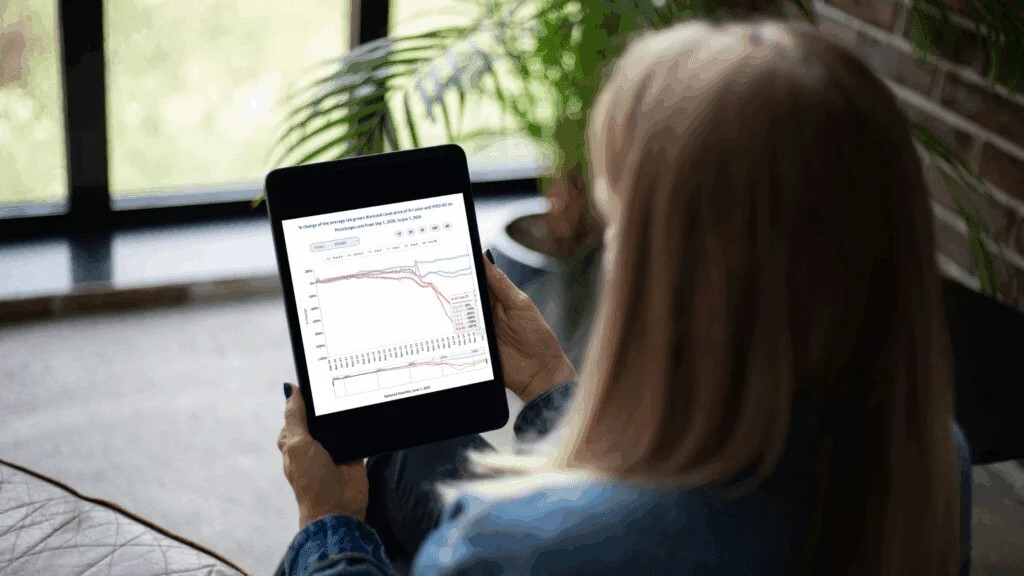
WHAT IS FLUORESCENCE?
Fluorescence is the glow sometimes seen when an object emits visible light. Some diamonds fluoresce when they are exposed to ultraviolet light. This can cause them to emit a bluish glow of varying intensities and less often so - a yellow, green or even a white glow.
It has been established over the years, through extensive testing that approximately 30% to 35% of all natural diamonds exhibit some degree of fluorescence when exposed to UV light. So it is fair to say that most diamonds found in nature do not fluoresce.
A diamond might fluoresce under a bright sun, at the dance floor or in other places where strong fluorescent or black light is present. Fluorescence is a temporary phenomenon and once the UV light source is turned off, the diamond stops fluorescing.
Diamond grading reports in general describe the intensity in 5 grades of fluorescence as: None, Faint, Medium, Strong and Very Strong. If the fluorescence level is Medium, Strong or Very Strong, the 'colour' of the fluorescence is also stated on these reports
If you’re still wondering what diamond fluorescence is, think about how ultra-violet light makes your whites look whiter and some of your teeth glow at the discotheque. In the same context, you may want to know that when a diamond fluoresces or radiates a glow, is that fluorescence good, bad or neutral for your diamond.
Here is an attempt to demystify some of the misinformation and conjecture surrounding fluorescent diamonds, so read on . . .
.webp)


.webp?width=342&height=301&name=download%20(7).webp)
.webp)
.jpg)